Complementation

"Francis would have to agree. Even though he was a physicist, he knew that important biological objects come in pairs." -Jim Watson in The Double Helix
At the other extreme, some scientists are intellectually engaged with one specific topic, spend many years finding the required tools and methods to solve the problem, and are unhappy when deprived of a chance to continue building on their life-time pursuit. Maurice Wilkins was focused on the study of DNA for many years. When an idea achieves a strong hold on the imagination of a scientist, the multitude of small choices perceived throughout life are channeled in one direction; the course of a career can then be understood in relation to that original captivating idea. The entire intellectual world of such a target-dedicated scientist becomes attached by strong bonds to the intellectual problem that defines their life’s work. The analogy in chemistry for such strong bonds is the covalent bond.
Pauling was the chemist who most clearly categorized chemical bonds and applied his knowledge of the properties of the few basic types of chemical interactions to the task of solving the structures of biological molecules. Pauling showed the world how this could be done. Could the young scientists of the next generation learn the lessons taught by Pauling and beat the old master to the solution of the DNA structure?

The base pairs are held together by hydrogen bonds. The phosphate backbones are held together by covalent bonds.
Just as both hydrogen bonds and covalent bonds are required for the function of DNA, Franklin and Wilkins had complementary approaches to scientific investigation that came together in such a way as to make possible the discovery of DNA's structure. Wilkins was the covalent bond of the Wilkins-Franklin complex, devoted to the mission of understanding the physical basis of the gene and willing to do anything, go anywhere, and use any technique needed to crack the problem. But just as there is not a single hydrogen bond in DNA and not only one type of covalent bond, Franklin and Wilkins were not alone as the discoverers of the structure of DNA.
Like Wilkins, Watson was also devoted to the idea that by examining in detail the physical components of living organisms, it would be possible to understand the biology of inheritance, to understand what passes from generation to generation to make offspring like the parents. Biochemists had started with living tissues and biological fluids and ground them up, extracted, purified and characterized the molecular components. The secret of life was slowly backed into a corner: the largest molecules resisted analysis and held on to the secret of replication, mutation, evolution and embryonic development. But what was the best way to solve this problem? Genetics? Biochemistry? X-ray diffraction?
Watson, the biologist, came at DNA from a different perspective than Wilkins, a physicist. Wilkins was among those physicists who had turned their attention to solving fundamental problems in biology. Watson was part of the new generation that started in fresh on the study of DNA just as it was being recognized as the genetic molecule.
In trying to deal with protein and DNA, classical biochemistry was restrained on all fronts, both theoretical and practical. The available armory of tools developed by biochemists served well for analysis of simple molecules that could be isolated from living organisms. However, the available tools were not up to the task of determining the details of protein and nucleic acid structure. These macromolecules presented new challenges and called out for new methods that could deal with their complexity and large size. Both new tools and new ways of thinking were needed to explore these important large molecules. Why did physicists begin to think that they could provide the required new thinking?

The Molecular orbitals of quantum chemistry.
The quantum revolution of the first quarter of 20th century had breathed new life into physics and provided the physical foundation needed by chemists like Pauling. Before quantum theory, chemistry limped along without a serious model of chemical bonds. Chemists were forced to think of chemical bonds as little hooks that linked adjacent atoms in molecules. Pauling and other chemists learned how to interpret covalent chemical bonds as the quantal orbitals of electrons shared between atoms. Along with the theoretical elucidation of chemical interactions, physics also made possible a whole new set of laboratory tools for probing molecular structure. Some physicists began to ask: could the new-found world view of a quantum world not only clear up the mysteries of chemistry but also fundamental biological mysteries?
If so, then there were two obvious possibilities for how this would play out. One possibility was that Pauling was on the right track. First straighten out the details of chemistry, then the identifiable chemical properties of the molecules in cells would account for Life. Not all physicists were willing to join Pauling on the slow slog of chemistry and felt that chemistry was boring. A more exciting possibility for some physicists was that some of the great mysteries of biology could be understood as direct consequences of quantum physics. For example, maybe the mystery of consciousness could be explained as some sort of quantum resonance in the brain. Such ideas have remained alive to this day.

Niels Henrik David Bohr, one of the founders of quantum theory.
Max Delbrück was one of Bohr’s students who turned from quantum physics to biology. Delbrück was interested in the genetic transmission of information. Life depends on the stability of genetic information passing from generation to generation in an unbroken chain for billions of years. The diversity of life arises from errors, changes in the old genetic instructions and production of new patterns. Starting with pea plants and fruit flies, biologists had tried to probe the basis of genetics and inheritance. How could genetic stability and genetic change both be accounted for in physical terms? Delbrück was among the first to speculate about the sources of energy and the types of physical events at the molecular level that might explain genetic change and stability.
Wilkins was among those scientists who were exposed to such issues by Schrodinger’s book “What is Life?” Schrodinger built on Delbrück‘s ideas about the physical process of mutation and made explicit the ideas of information storage in the form of molecular structures. In particular, he described the theoretical idea that genetic information could be encoded in the form a sequence of molecular subunits inside extended “aperiodic crystals” (we would say “polymers”). For those like Wilkins who sought to move beyond this theory, the question became, which polymer? Protein? DNA? Both? Protein and DNA were the two known components of chromosomes and it was clear that chromosomes carried the growing number of genes known since Mendel’s work with pea plants and exposed with increasing detail by mutated fruit flies. But just identifying genes and gene mutations was not enough. Biochemistry allowed identification of the atomic constituents of protein and DNA, but a technique was needed to reveal the details of the molecular structure. X-ray diffraction was an obvious choice to fulfill this need, but nobody knew how to carry out the work. Proteins were hopelessly complex and DNA was hopelessly fragile and difficult to isolate in its intact form for crystallization. X-ray diffraction analysis of molecular structure depends on having crystals of the target molecule. Which laboratory would first find a way to reveal the molecular structure of the genetic molecule?

Mutant eye color phenotypes in Drosophila.
Immediately after World War II, the physicist John Randall turned his attention to biology. Randall had helped win the war with the invention of radar and he was rewarded with the funds needed to start a new biophysics laboratory. Wilkins joined Randall’s new biophysics laboratory in London and started to apply physical techniques to probe the genetic material of chromosomes. It had been shown that X-rays could cause mutations in fruit flies. At this time, the young Jim Watson was working on his PhD research in a lab where ultraviolet light and X-rays were being used to probe genetic transmission by viruses. The study of viral genetics was the brain child of people like Delbrück who guessed that viruses might be very simple, maybe even “naked genes”. Wilkins was mentally prepared to try anything as a probe of the genetic material. Could concentrated sound waves fracture chromosomes? Could ultraviolet light, strongly absorbed by DNA, reveal the mechanics of DNA inside cells? Wilkins used ultraviolet light in parallel studies of intact cells and purified nucleotide bases, the structural components of DNA. Wilkins also applied ultraviolet light to the study of a virus. Wilkins moved on to try X-rays, including X-ray diffraction studies of DNA inside cells that were rich in DNA. Wilkins was one of the few people in the world focused on probing the structure and function of DNA, but his work had a feel of desperation expressed by the formula “I’ll try anything”.
At this time, another English physicist who was moving into biology, Francis Crick, visited Randall’s lab, started an enduring friendship with Wilkins, and ended up working on x-ray diffraction studies of protein in the Cambridge lab of Max Perutz.
At this time, it was not clear that DNA was the genetic molecule, but evidence pointed in that direction. Avery had shown in 1944 that purified DNA could transfer a genetic change in bacteria. But other ideas suggested that DNA was a “stupid” molecule. DNA seemed to have a simple biochemistry and it had been suggested that it might be a polymer with a simple repetition of its four nucleotide subunits. Maybe all that DNA did inside a chromosome was provide a scaffold for the more important proteins. In the late 1940s, these were the theories being taught to students like Watson.

Adenosine monophosphate.

Thymidine monophosphate.
But at the same time, members of the small “Phage Group”, those labs using bacterial viruses to study genetics, were aware of experiments with electron microscopy that suggested these viruses had two parts, only one of which carried their genes into the cells they infected. Experiments were underway to test biochemically if it was the viral DNA or the viral protein that was transferred into infected cells to carry the genetic message. In addition to Avery’s result, another piece of biochemical knowledge also spoke quietly against the “stupid tetranucleotide” view of DNA. Erwin Chargaff had found that while the four nucleotide subunits of DNA were present in roughly equal amounts, they were not there in exactly equal amounts. Did the variations from species to species indicate differences in the genetic instructions of different species? What came to be known as the Chargaff ratios, experimentally determined ratios of the nucleotide subunits of DNA, indicated that the amount of guanine is equal to cytosine and the amount of adenine is equal to thymine. Mysteriously, while the absolute amounts of each nucleotide varied from species to species, in all cases the amount of A was equal to the amount of T and the amount of G was equal to the amount of C. What might this complementation mean? Was it saying that DNA was interesting, that it was the genetic molecule?
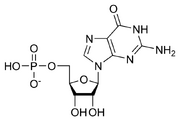
Guanosine monophosphate.

Cytidine monophosphate.
Although Randall’s biophysics laboratory was so new that its building was still under construction, students started to arrive and join in the grand effort to use the tools of physics to solve biological mysteries. Ray Gosling began doing x-ray diffraction work with Randall and others, doing experiments on sperm, the heads of which are basically nuclei, tightly packed with chromosomes and providing a concentrated source of DNA. The first experiments were crude and accomplished with the kind of cobbled together equipment that many experimental efforts often start with.
In March of 1950, Rosalind Franklin was looking for a job in London. Franklin had published an X-ray crystallography article in the journal Nature in January 1950, reporting results of post-doctoral work she was then performing in Paris. She was hoping to trade her growing scientific reputation for a job in England. She was introduced to Randall at King’s College. Randall was actively growing his laboratory and needed people like Franklin who were trained in X-ray crystallography. They began to work together to try to arrange for a research fellowship that would support Franklin in Randall’s lab. While the fellowship application processing went on, Franklin continued her work in Paris. That work concerned X-ray diffraction studies of coal and carbon. Randall thought that Franklin’s experience made her well suited to start work on experiments using X-ray diffraction to study proteins.
Wilkins was developing an interest in finding ways to compare X-ray diffraction results for DNA inside intact cells and chemically purified DNA. Did isolation and purification of DNA change its shape, rendering X-ray diffraction studies of purified DNA useless? In May of 1950 Wilkins fortuitously came upon a source of purified DNA when a visiting scientist, Rolf Signer, provided him with a sample of purified DNA that was more intact than any other that was then available. It is very easy to break the long thin DNA molecules isolated from chromosomes while trying to purify them, but the DNA from Signer’s lab was largely intact.
The type of X-ray diffraction data collected for DNA in 1950
Wilkins started working with the purified DNA and found that in solution it was highly viscous (like snot) and that he could use a glass rod to pull thin threads of it up out of the solution. He began to perform ultraviolet microscopy on Singner's DNA and the results indicated that The DNA was present in ordered arrays. Wilkins and Gosling performed X-ray diffraction experiments on the DNA threads. Wilkins and Gosling found that the threads diffracted X-rays. They were more suitable for X-ray diffraction studies than any previously tested form of DNA. Still, Wilkins was worried that the X-ray diffraction pattern obtained with the purified DNA seemed very different from what had previously been observed for DNA in cells. Were the old data from cells misleading, should they be ignored? Was the new X-ray diffraction pattern for the purified DNA an artifact resulting from purification? Would detailed analysis of the new X-ray diffraction pattern reveal a structure that was meaningless for biology and the function of DNA in living organisms?
Early in June, Franklin returned to King’s College for an interview with the sponsor of the fellowship she hoped to be awarded. Randall and Wilkins had recently discussed what needed to be done to follow up with detailed X-ray diffraction analysis of the purified DNA. It was clear that King’s College needed better X-ray diffraction equipment and technical expertise in the form of a scientist with X-ray diffraction experience. Wilkins and Gosling met with Franklin and showed off their X-ray diffraction images of the purified DNA.
Franklin asked to see their equipment and was appalled by the primitive nature of the X-ray diffraction facilities at King’s College. Randall had already told her that the lab would soon move out of its temporary digs and he had described to Franklin what the new facilities would be like in the building that was under construction. Wilkins explained to Franklin that there was no shortage of money for new equipment and that he was already making arrangements for a new X-ray tube that would allow for collection of better X-ray diffraction images of the thin DNA fibers. Franklin gave Wilkins suggestions for what additional equipment would be needed to continue the DNA work, and returned to Paris.
Later that month, the fellowship was awarded to Franklin for support of X-ray diffraction work in Randall’s lab. Franklin needed more time in France, so Randall arranged to have the fellowship funding start in early 1951. Wilkins and Franklin exchanged several letters and Franklin communicated to Wilkins how to move ahead with preparations for using the new X-ray tube. Wilkins had the King’s College machine shop start work on components for the new equipment Franklin wanted in place when she arrived.
In November, Franklin sent a letter to Randall listing equipment that would be needed for X-ray diffraction work when she moved from Paris to King’s College at the start of the new year. Randall wrote back to Franklin confirming that she should switch from the original plan to work on protein and instead follow up on the results of Wilkins and Gosling indicating that good results could be obtained with Signer's purified DNA.
Early in 1951 Franklin arrived at Kings College to start her new job. Randall arranged for a meeting with Franklin, Gosling and a theoretician named Alec Stokes. Randall described Franklin, Gosling, Stokes and Wilkins as his new DNA research team. Wilkins was out of town, but Randall explained to Franklin that upon his return Wilkins would work with Gosling to teach her how to work with the DNA threads. Wilkins and Stokes would collaborate with Gosling and Franklin to fit the DNA X-ray diffraction results into the existing body of knowledge about DNA. Randall also explained that he was working towards the goal of having another team perform chemical studies on the DNA sample that was so good for X-ray work.
Franklin and Gosling had some fine tuning to do on the new equipment that Wilkins had brought together, but by the time Wilkins was back, Franklin and Gosling had taken their first diffraction picture of DNA with new X-ray tube and the old camera. Franklin, Gosling and Wilkins passed back and forth a print of the new picture. It was clear from the image that the new X-ray tube was a big step forward from the type of tube that Watkins and Gosling had used before. They discussed how to make sure that the DNA sample being bombarded with X-rays would stay moist while as little air as possible would be in the X-ray path.

X-ray diffraction image for DNA in cells.

X-ray diffraction image for purified DNA (wet form).
While the components of the new camera were produced by the machine shop, Franklin had plenty of work to do writing up for publication more results from her work in Paris. By April the new equipment was ready and Franklin and Gosling began to use the new setup. With the new system for controlling the moisture content of the DNA sample, they found that the DNA fibers could switch reversibly between to states of hydration. They took images of a single fiber under “wet” and “dry” conditions and showed that the X-ray diffraction results for the two forms were dramatically different. Franklin named the two patterns “A” and “B”.
Wilkins was very relieved that by carefully keeping the DNA wet during the X-ray imaging, Franklin could produce a pattern simiar to what was found for cells. The fact that DNA molecules could reversibly shift from the A to the B form suggested that there might not be much difference between the wet and dry DNA conformations.
Alec Stokes and Franklin both felt that the “B” pattern showed signs of arising from a helix. Stokes agreed to try to devise a theoretical account of how a helical molecule should diffract X-rays. Franklin was much more interested in the diffraction pattern of the A-form because its many reflections could provide more details about the positions of atoms in DNA.
Randall had been invited to a meeting in Naples where research on nucleic acids would be discussed. He asked Wilkins and Franklin to go to Naples and describe their results. Franklin explained that she was planning to attend another meeting in June that would deal with X-ray crystallography. Randall agreed that it would benefit Franklin to attend the nucleic acids meeting also and that Wilkins could benefit from the attending the crystallography meeting.
In May of 1951, Franklin and Wilkins attended the meeting in Naples. In the time slot for Randall, Wilkins first described his results from the previous year then Franklin showed the latest images. Wilkins concluded the presentation by talking about the fact that the B form seemed to indicate a helical structure for DNA. In the audience was Jim Watson. At the end of the session there was a clot of senior scientists around Wilkins. Seeing an opening, Watson moved forward to talk to Franklin. He introduced himself as a biologist who was interested in proving that DNA was the genetic molecule. Franklin asked how he proposed to do so and Watson, Franklin and Wilkins ended up discussing the matter over dinner. Watson told about the experiments that were underway to test if it was DNA or protein that carried viral genes into bacteria. Watson and Wilkins talked a lot about viruses while Franklin tried to make sense of the conversation. Her limited biology background was a serious handicap, but as Randall had predicted, she was learning a lot by being at the meeting.
Franklin wondered how much of what Watson was saying could be trusted. If he was right about there being good evidence that DNA was the molecule that carries genetic instructions, then she had been lucky enough to become part of one of the most important experiments in biology.